|
We have learned all the skills we need to be ready for our quiz on Tuesday. We will continue practicing and reviewing Monday then take our assessment the following day.
GOLD TAPE QUESTION: Gilbert N. Lewis is the scientist who developed the diagrams we still use today to draw molecules and their electrons. Lewis also developed what are now known as "Lewis Acids" and "Lewis Bases" Two pieces of gold tape is up for grabs this week! 1) What is a Lewis Acid? 2) What is a Lewis Base?
0 Comments
Yesterday we discussed the Octet rule and today we used the octet rule to create Lewis diagrams of both atoms and molecules. Use the videos below to review these concepts.
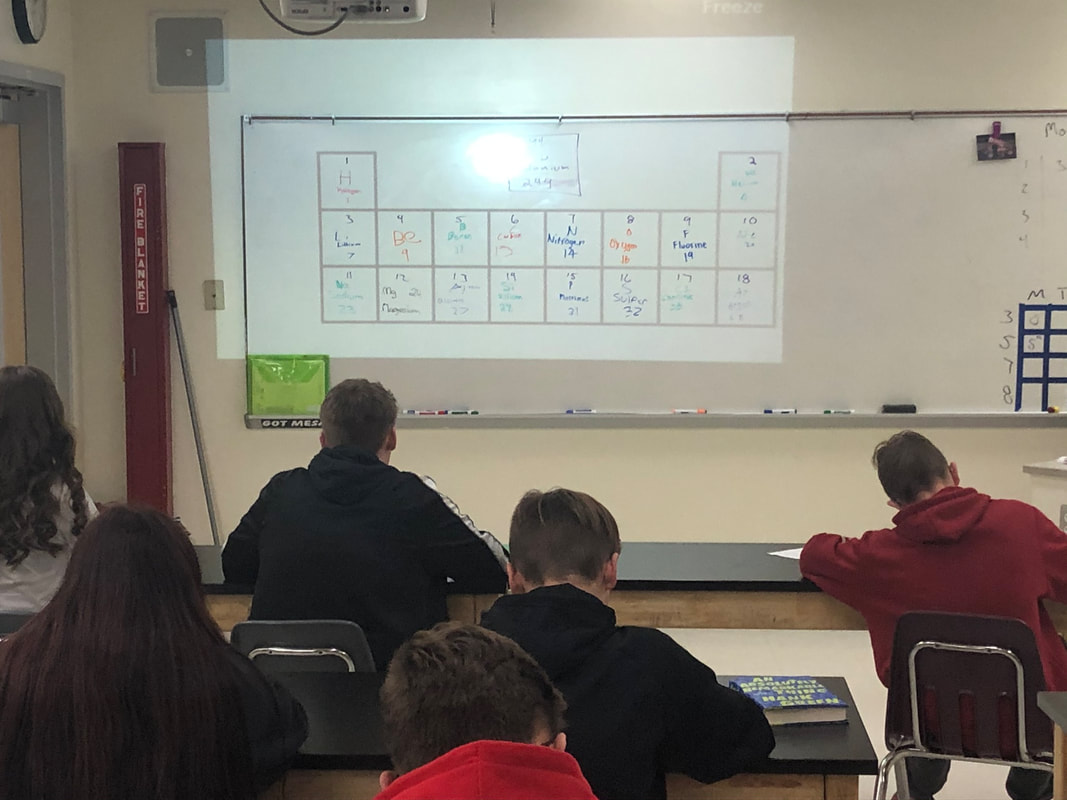
Today we built periodic tables of the first 18 elements and learned about the Bohr model of the atom. Tomorrow we will learn about the Lewis Dot structure and continue practicing diagramming atoms. We will have our final chemistry assessment this Friday.
On Thursday you will have an exam covering the Law of Conservation of Mass and balancing chemical equations. Use the videos below to help you study for your exam.
Watch the video below and practice balancing equations along with the video. You should pause and try to solve them yourself before watching the answer. In class today we took a quiz on balancing chemical equations. Your GOLD TAPE QUESTION for this week is about a family of molecules we haven'y yet discussed in class. Click THIS LINK to learn about Forever Chemicals answer the following question: What are "Forever Chemicals" and what are scientists trying to do to them?
Gold Tape Question: Methane has one carbon, ethane has two, propane has three and butane has four. What is the name of a hydrocarbon with TWELVE carbons?
|
AuthorMr. Powell is a High School Science Teacher in Western Colorado. Archives
May 2024
Categories
All
|


 RSS Feed
RSS Feed